Research
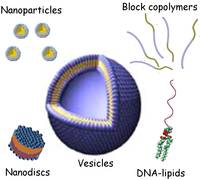 Much of our research is inspired by the functional roles of biological membranes in regulating the spatial compartmentalisation and transport of chemical information within cells, tissues and organisms. We aim to combine natural biomolecules with synthetic polymers and particles to engineer novel materials as well as to use physical science approaches to improve our understanding of the structure and function of membranes in biology. These goals lead towards applications in drug delivery, toxicology and nanoreactors, while moving us closer towards realising the bottom-up design of synthetic biological cells in the lab.
Much of our research is inspired by the functional roles of biological membranes in regulating the spatial compartmentalisation and transport of chemical information within cells, tissues and organisms. We aim to combine natural biomolecules with synthetic polymers and particles to engineer novel materials as well as to use physical science approaches to improve our understanding of the structure and function of membranes in biology. These goals lead towards applications in drug delivery, toxicology and nanoreactors, while moving us closer towards realising the bottom-up design of synthetic biological cells in the lab.
Membrane Biophysics
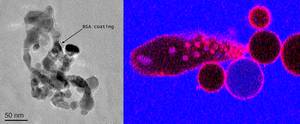 We have a broad interest in understanding the properties and functions of biological membranes using minimal membrane models to provide mechanistic insights. Primarily we work on unsupported vesicle membrane models from ensemble spectroscopic analysis of sub-micron vesicles to advanced microscopies to study cell-sized microscale vesicles at the single vesicle level. We also collaborate widely with other membrane biophysics groups in Leeds to complement our work using additional techniques and membrane models. We apply multiple approaches to investigate the membrane structure, mechanical and dynamical properties and how these are affected by interactions with extraneous biological or synthetic matter.
We have a broad interest in understanding the properties and functions of biological membranes using minimal membrane models to provide mechanistic insights. Primarily we work on unsupported vesicle membrane models from ensemble spectroscopic analysis of sub-micron vesicles to advanced microscopies to study cell-sized microscale vesicles at the single vesicle level. We also collaborate widely with other membrane biophysics groups in Leeds to complement our work using additional techniques and membrane models. We apply multiple approaches to investigate the membrane structure, mechanical and dynamical properties and how these are affected by interactions with extraneous biological or synthetic matter.
We are interested in a wide range of biophysical questions related to membranes. Recent work has included: (i) understanding the regulation of activity of ESCRT membrane remodelling complexes that deform the membrane and bud off new vesicle compartments by a topologically unique mechanism; (ii) uncovering the mechanisms for specificity and potency of membrane disrupting anticancer peptides; (iii) characterising the interactions of inorganic nanoparticles with lipid bilayer membranes, relevant to their potential toxicology and their applications within biomedical therapies.
Hybrid Vesicles
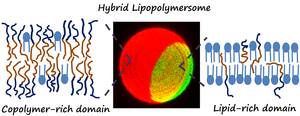 We aim to make novel functional materials by making hybrid composites of natural lipids with synthetic polymers. Our aim is to synergistically enhance the properties of self-assembled vesicles for technological applications by combining the advantages of different material systems. In the case of hybrid lipid - block copolymer vesicles, this brings together the natural biocompatibility and biofunctionality of the lipid with the enhanced stability, toughness and tuneability of polymer vesicles.
We aim to make novel functional materials by making hybrid composites of natural lipids with synthetic polymers. Our aim is to synergistically enhance the properties of self-assembled vesicles for technological applications by combining the advantages of different material systems. In the case of hybrid lipid - block copolymer vesicles, this brings together the natural biocompatibility and biofunctionality of the lipid with the enhanced stability, toughness and tuneability of polymer vesicles.
Particular success has been achieved in reconstitution of integral membrane proteins into hybrid vesicles. Lipids are required to provide a native-like membrane environment to reconstitute high functional activity of these proteins, but the polymers also provide significant advantage in prolonging the functional durability of the membrane protein. This has led to some systems with order of magnitude increases in functional lifetime with >20% of the initial activity of the membrane protein still retained after 18 months storage at 4℃ following reconstitution.
Nanomedicine
We are interested in applying concepts in soft and biological matter to unmet medical needs. Our primary activity in this area is in formulation of therapeutics in nanoparticulate drug delivery systems. Most of these are designed as long-acting parenteral delivery systems with tuneable drug release rates, but we also have some systems designed for environment-responsive triggered release. Applications of particular interest are currently in oncology and surgical analgesics, but the disease-specific expertise come through collaboration and so we are always keen to turn our hand to new disease indications where our systems have the potential to meet a specific need.
A second area of biomedical interest is in regenerative medicine where we are helping develop injectable hydrogels to restore natural tissue mechanics that have been lost through local degeneration. The ability to tune hydrogel mechanics to match the tissue type of interest is important for generalising this approach. However our core focus at present is regeneration of the nucleus pulposus as a treatment for chronic back pain.
The Artificial Cell
The controlled compartmentalisation and transport of chemical information is central to the function of living organisms. We have a long term and highly ambitious aim of developing an artificial cell from bottom-up self-assembly of its constituent components. To make such an approach adaptable and broadly generalisable for a wide range of synthetic biology applications, an integratable toolbox of relevant fabrication strategies is required. We are applying our expertise in understanding and controlling reconstituted membrane-based systems to develop structures with: (i) controlled compartmentalised membrane architectures analogous to organelles in a eukaryotic cell; (ii) enhanced stability and durability of an artificial cell by combining natural and synthetic building blocks; (iii) encapsulation of functional chemistries, in particular those which mimic feedback-response in natural biochemical systems and facilitate communication of signals between distinct membrane-bound compartments. Our goal is to create new functional biologically-based devices and materials while, along the way, discovering new insights into how biological systems function.
